标准品/甲醇中21种抗感染类药物混标/SN/T 2579-2010,100μg/mL
产品编号西域质检-TM17722
Cas号
纯度/浓度100μg/mL
储存温度冷冻
增值税发票√顺丰快递√订货电话:18601927057
- 中文名称:标准品/甲醇中21种抗感染类药物混标/SN/T 2579-2010
- 英文名称:
- CAS NO:
- 分子式:
- 分子量:
基本信息
This certificate is designed in accordance with ISO 17034 and ISO Guide 31. This reference material (RM) was designed,produced and verified in accordance with ISO/IEC 17025, ISO 17034 and a registered quality management system ISO 9001.
冷冻(-18±5)℃,置于阴凉处
| CERTIFIED | |||||
|
Component No. (组分数) |
Peak Sequence (出峰顺序) |
Component (组分) |
CAS No. |
Concentration (μg/mL) (浓度) |
Relative Expanded Uncertainty(%) (k=2) (相对扩展不确定度) |
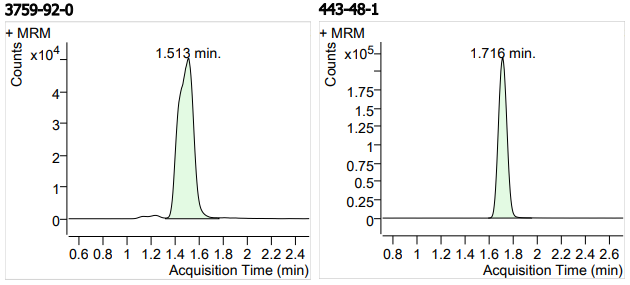
| 1 | 1 | Furaltadone Hydrochloride1 (呋喃它酮盐酸盐) | 3759-92-0 | 100(free base) | 3 |
| 2 | 2 | Metronidazole (甲硝唑) | 443-48-1 | 100 | 3 |
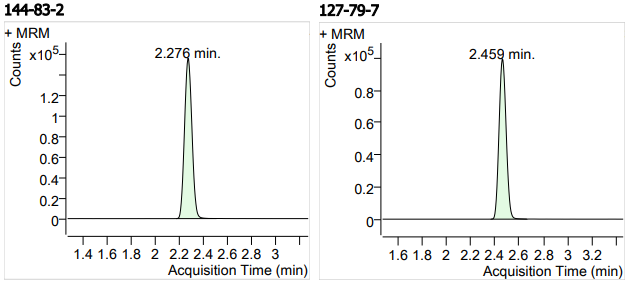
| 3 | 3 | Sulfapyridine (磺胺吡啶) | 144-83-2 | 100 | 3 |
| 4 | 4 | Sulfamerazine (磺胺甲基嘧啶) | 127-79-7 | 100 | 3 |
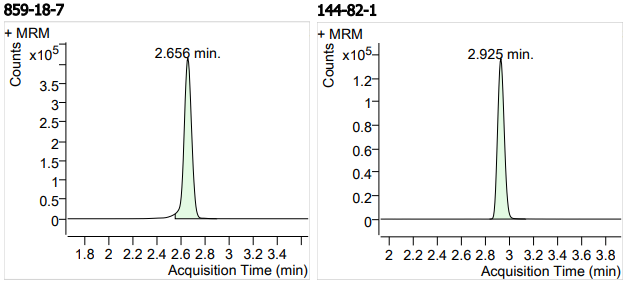
| 5 | 5 | Lincomycin Hydrochloride2 (盐酸林可霉素) | 859-18-7 | 100(free base) | 3 |
| 6 | 6 | Sulfamethizole (磺胺甲噻二唑) | 144-82-1 | 100 | 3 |
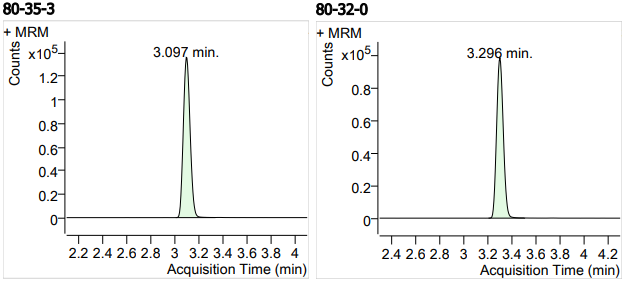
| 7 | 7 | Sulfamethoxypyridazine (磺胺甲氧哒嗪) | 80-35-3 | 100 | 3 |
| 8 | 8 | Sulfachloropyridazine (磺胺氯哒嗪) | 80-32-0 | 100 | 3 |
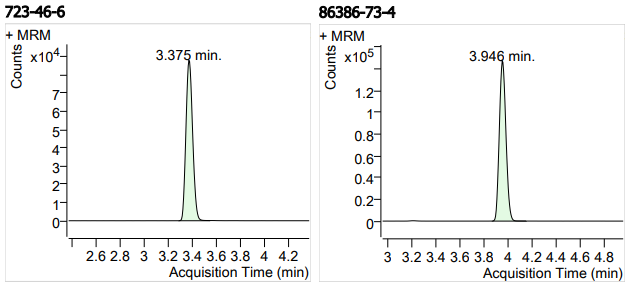
| 9 | 9 | Sulfamethoxazole (磺胺甲基异噁唑) | 723-46-6 | 100 | 3 |
| 10 | 10 | Fluconazole (氟康唑) | 86386-73-4 | 100 | 3 |
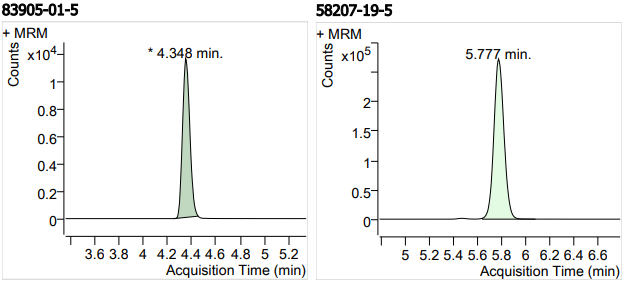
| 11 | 11 | Azithromycin (阿奇霉素) | 83905-01-5 | 100 | 3 |
| 12 | 12 | Clindamycin Hydrochloride 3 (盐酸克林霉素) | 58207-19-5 | 100(free base) | 3 |
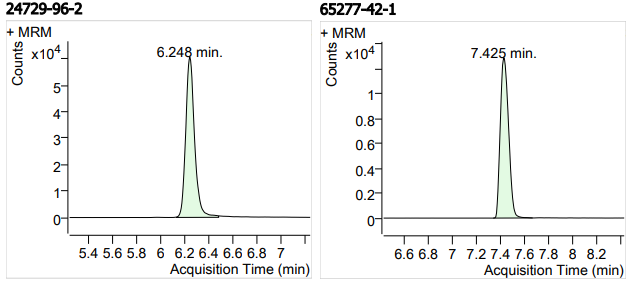
| 13 | 13 | Clindamycin Phosphate (克林霉素磷酸酯) | 24729-96-2 | 100 | 3 |
| 14 | 14 | Ketoconazole (酮康唑) | 65277-42-1 | 100 | 3 |
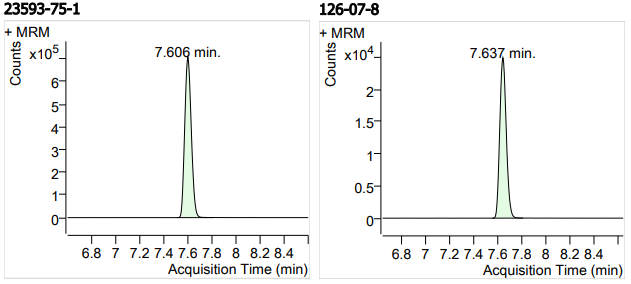
| 15 | 15 | Clotrimazole (克霉唑) | 23593-75-1 | 100 | 3 |
| 16 | 16 | (+)-Griseofulvin ((+)-灰黄霉素) | 126-07-8 | 100 | 3 |
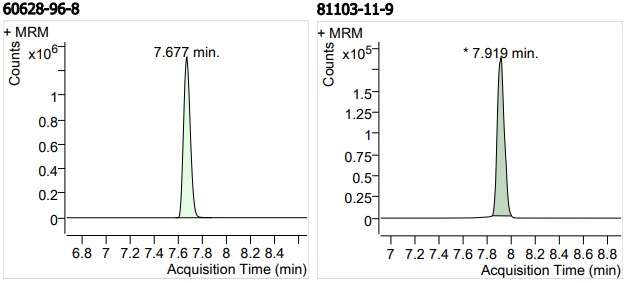
| 17 | 17 | Bifonazole (联苯苄唑) | 60628-96-8 | 100 | 3 |
| 18 | 18 | Clarithromycin (克拉霉素) | 81103-11-9 | 100 | 3 |
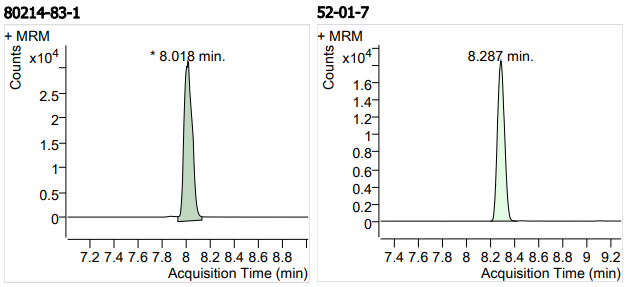
| 19 | 19 | Roxithromycin (罗红霉素) | 80214-83-1 | 100 | 3 |
| 20 | 20 | Spironolactone (螺内酯) | 52-01-7 | 100 | 3 |
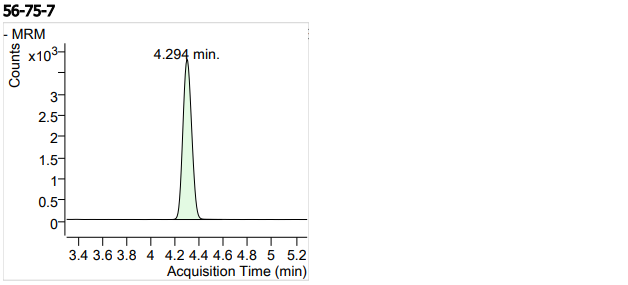
| 21 | 21 | Chloramphenicol4 (氯霉素) | 56-75-7 | 100 | 3 |
This RM is intended for use in a laboratory as a calibration and quality control standard or in method development for analytical techniques.
The certified value(s) and uncertainty(ies) are determined in accordance with ISO 17034 with an 95% confidence level (k=2). Uncertainty is based on the Total Combined Uncertainty, including uncertainties of preparation, purity of neat materials, homogeneity, stability testing.
The balances used for gravimetric measurements are calibrated with weights traceable to the national standards. The calibration of the balances is verified annually by an external accredited calibration service. This analysis method has been verified using an approach consistent with ISO 17034:2016 & ISO 17025:2017.
Random replicate samples of the final packaged RM have been analysed to prove homogeneity consistent with ISO 17034.
The RM should be stored in the original sealed bottle at the indicated temperature.
| CERTIFICATE ON | QC SIGNATURE | |
| 2023-Jan-03 |

|
RM Release |










 浙公网安备 33010802013077号
浙公网安备 33010802013077号